

|
|
|
|
Our group, Biomedical Molecular Microscopy, studies molecular interactions in cell signalling of membrane proteins and cytosolic messengers by fluorescence resonance energy transfer (FRET) microscopy, single-molecule microscopy, and dynamic confocal microscopy. These approaches are made possible by the building of custom wide-field and confocal microscopes, capable of ratiometric FRET, fluorescence recovery after photobleaching (FRAP), fluorescence correlation spectroscopy (FCS), and single-molecule tracking. These microscopes allow the detection of low, endogenous levels of proteins in and on living cells. We focussed the past year on Bone Morphogenetic Protein (BMP) signalling mechanisms, on interleukin-5 (IL-5) clustering on living eosinophils, and on Ca2+ signalling. Other projects include ion channels, platelet signalling, G-Protein Coupled Receptors, and model membrane systems. We also develop microscopes and methods to study single-molecule interactions in living organisms. |
|
|
|
|
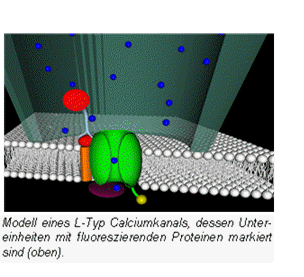
Imaging of calcium signaling mechanisms
Calcium is one of the most important second messengers of cell signaling and regulates specific events such as muscle contraction. Although, much is known about the role of calcium in the cell, remaining questions about mediators of calcium are crucial for our understanding of calcium signalling. |
|
|
|
|

Interleukin-5 ReceptorTargeting asthma by interference of cytokine networks is promising for prevention. IL-4, -5 and -13 expressed by Eosinophilic granulocytes play a key role in TH2-type immune response dominating the exacerbation of atopic diseases. The low endogenous expression of IL-receptors (~1000 receptors/cell) on Eosinophils requires utmost-sensitivity imaging and the bright QDots because of the high Eosinophilic background autofluorescence. IL-5 receptor common ß-chain (IL-5Rbc) dynamics were visualized on the surface of HL-60 cells (differentiated to eosinophils) by QDots coupled to specific antibodies. IL-5Rbc’s on these cells demonstrate two patterns of confined motion due to membrane flow and cytoskeletal restructuring. The receptors show a high complexation stoichiometry indicating that the crystallographic picture of IL-5Rbc as a stable intertwined homodimer is present on the surface of living Eosinophils, but on living cells, receptor complexes of higher order might be necessary for signaling. |
|
|
|
|

Bone Morphogenetic Protein SignallingThe BMP signalling system regulates growth and differentiation and is important for tissue engineering. BMP and BMP receptors are implicated with diseases as Preliminary Pulimary Hypertension, Juvinile Polyposis, breast cancer, colon cancer, and other forms of cancer. BMPs, part the of Transforming Growth Factor (TGF-ß) super-family, regulates through two types of receptors, BRI and BRII. The BMP ligand binding event controls signalling and is regulated by either binding to preformed homodimer complexes of BRI, signaling the p38 Map Kinase pathway or to preformed heteromeric (BRI and BRII) complexes for the Smad dependent pathway. A more precise picture of the receptor signalling can now be described by observing individual receptors on the surface of the biologically relevant cells. Individual BRII receptors show patterns of mobile and immobile diffusion that correspond to preformed complexes. These studies also indicate that membrane micro-domains and the cytoskeleton are involved in the regulation of complex formation. |
|
|
|
|

Smad 1/4 SignallingThe Smad signalling pathway, one mediator of BMP signalling, influences cell growth, differentiation, adhesion, migration, and also carcinogenesis and immune responses. Smads mediate the signal from the membrane into the nucleus. Smad1 is phosphorylated upon Bone Morphogenetic Protein (BMP) stimulation and interacts to form a complex with Smad4. This complex translocates into the nucleus and regulates transcription of target genes. Biosensors of Smad1 and Smad4 fused to cyan and yellow fluorescent proteins allowed us to determine the rate-limiting steps of this signaling cascade by FRET microscopy. We were able to visualize rate-limiting delays between BMP-4 activation, Smad1 phosphorylation, and complexation. Further experimentation indicated the delays are influenced by the MH1 domain of Smad1. The Smad biosensors give new insights into the BMP–Smad1/4 signaling process and provide a powerful tool for rapid evaluation of Smad activation. |
|
|
|
|
|
Imaging of platelet adhesion signalingPlatelet adhesion to fibrinogen is an important event in wound healing and cardiovascular medicine. Adhering platelets generate `outside-in’ signals from the adhering integrin protein inward into the cell. This is an under-explored facet in platelet signaling. Activated integrins cause profound cellular cytoskeletal rearrangement and spreading due to signalling of Src-kinases and multiple downstream response proteins. One of these proteins, Csk, inactivates Src. Previously, it has been shown biochemically that Csk forms a complex with the aIIbß3 integrin and Src. We have now elucidated and observed the cellular dynamics of the Src inactivation by Csk by live cell FRET microscopy. This result is a first step in understanding how the ‘outside-in’ signal transfers throughout platelets. 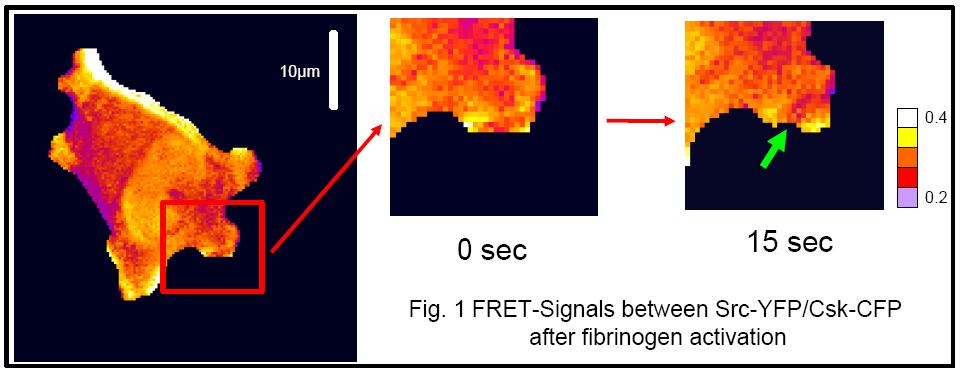
|
|
|
|